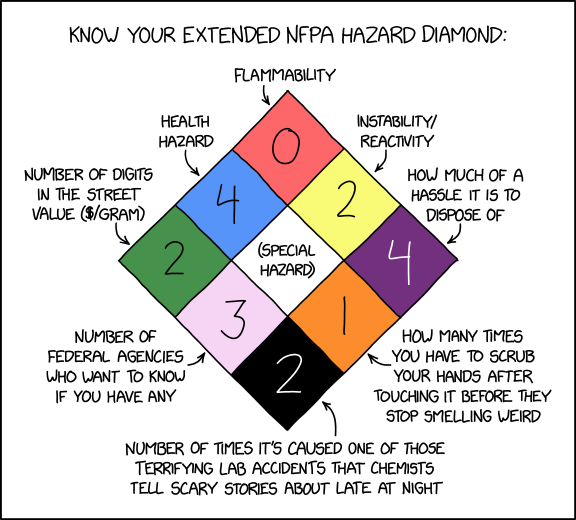
Extended NFPA Hazard Diamond
With most labs, the hushed horror stories are about something like dimethylmercury or prions, but occasionally you'll get a weird lab where it's about the soda machine or the drop ceiling.

With most labs, the hushed horror stories are about something like dimethylmercury or prions, but occasionally you'll get a weird lab where it's about the soda machine or the drop ceiling.
This comic depicts an extension of the National Fire Protection Association's NFPA 704 Standard System for the Identification of the Hazards of Materials for Emergency Response "fire diamond" emblematic insignia used to warn about the properties of hazardous substances inside a building, vehicle, room, cabinet, or container that are important during an emergency or accident, such as a fire, earthquake, spill or leak, bringing the diamond from 2x2 squares to 3x3 by adding five variously useful and humorous squares along the bottom edges. NFPA Hazard Diamonds have also been mentioned in 868: Nolan Chart.
The numbers in a normal NFPA 704 diamond do not specify values of substances' properties, but rather broad categories designating characteristics of the substances of greatest interest to first responders and hazardous materials cleanup crews. Randall's expanded diamond breaks with this convention, with several squares (Lilac, Orange, and Black) denoting absolute values, and one square (Green) denoting an economic value. This could very easily lead to documentation update headaches, especially since the Green square is mostly determined by supply and demand, and the Lilac square is linked to political outcomes. See explanation for each field in the extended square below in the table.
The only easily identifiable substance which could likely meet the specific insignia numbers shown in the comic is thionyl chloride (SOCl2), a chlorinating reagent and solvent regulated as a chemical weapons precursor and sometimes used in the production of methamphetamine, which would also be represented with the
Wsymbol inside the white square, indicating reactivity with water.The title text (which references "scary stories" of the Black square) refers to dimethylmercury and prions. Dimethylmercury, C2H6Hg, is an organic form of mercury with an NFPA score of 4-4-3 (contact can be fatal, will burn below 73° F (22 °C), will combust if put under pressure). In 1997, an American chemist, Karen Wetterhahn, died 298 days after a few drops of C2H6Hg on her latex gloves were absorbed into her hand through the gloves, causing fatal mercury poisoning. Despite her having followed all safety protocols of the time, it was not then understood that the chemical was so toxic, nor that latex was so permeable to it. Prions are misfolded proteins that are responsible for a number of neurodegenerative diseases, including mad cow disease and chronic wasting disease in non-human animals and Creutzfeldt-Jakob disease in humans. These would indeed be the kind of substances that would scare those working with them in their labs; if an accident occurred, the results could be calamitous. See for example the case of Émilie Jaumain, a lab technician who died after accidentally coming into contact with prions in mouse tissue.
But a few labs have apparently had accidents involving a soda machine or dropped ceiling. The latter may be a reference to the death of Janet Parker: One inquiry found that she was infected with smallpox when a sample traveled upward from a lab on the floor below hers; however, other investigations have challenged that finding. There are occasional instances of vending machines causing injury or death, usually caused by people trying to shake or tilt the machines to get product out and having the machine tip and fall on them. On average, a couple of Americans per year are killed in this way. Reagents obtained in this way tend to have more impurities than those usually used in labs, but are relatively safe to shake.
Extended diamond
Square Color Comic text Explanation Real NFPA 704 diamond square and number meanings Top Red Flammability (0) Denotes flammability. 0 indicates "materials that will not burn." Top Left Blue Health Hazard (4) Denotes the danger that the substance(s) pose to living beings in ways other than flammability and reactivity. 4 indicates that "Very short exposure could cause death or serious residual injury even though prompt medical attention was given." Top Right Yellow Instability (2) Denotes how stable the substance(s) are when exposed to water, heat, shock, air, or other substances. 2 indicates that "Normally unstable and will readily undergo violent decomposition but does not detonate. Also: may react violently with water or may form potentially explosive mixtures with water." Center White (Special Hazard) The standard's "Special Notice" field may contain a symbol denoting additional information about the substance(s), e.g., OX for oxidizers, SA for simple asphyxiant gases such as nitrogen and helium, and Wfor substances which react dangerously with water.After this point, all squares are made up by Randall. Center Left Green Number of digits in the street value ($/gram) (2) Describes the order of magnitude of the price (in USD) of one gram of the substance when sold illegally and informally. This is done on a logarithmic scale, with a '1' selling for $9/gram or less, a '2' selling for $10-$99/gram, and so on. As such this is the first of several squares where the number may presumably go to 5 or above (which is not allowed on the original Blue/Red/Yellow squares, as they do not denote strict numerical values). That said it's not immediately clear how substances which command <$1/gram would be handled. Randall's example substance apparently sells for tens of dollars per gram (which would be similar to most common illicit drugs).
Center Right Dark Purple How much of a hassle it is to dispose of (4) While many things can be thrown in the trash with no additional procedures, substances that merit an NFPA 704 square often require additional procedures to avoid significant danger, damage to the environment, or hefty dumping fines. Biohazards that may carry diseases are often disposed of in special containers, and nuclear materials are notoriously difficult to safely dispose of. This square would be at least theoretically useful, though not as much as the actual disposal guidelines; simply knowing it has a non-zero value would be enough for anyone with the slightest respect for safety to avoid throwing the substance into a normal trash can. If the numbering here follows the scheme of the real categories, Randall's example substance is about as hard to dispose of as it gets. This matches the substance's rating of 4 for Blue and 2 for Yellow. Presumably it requires highly specialized handling or processing, and may also very bulky or awkward to store.
Bottom Left Lilac Number of federal agencies who want to know if you have any (3) In many countries, including Randall's home country, the USA, the government has agencies dedicated to controlling or limiting the use of regulated substances, due to their use as drugs, weapons, harm to the environment, etc. While any given substance might be of interest to one agency, something that is both an environmental hazard and a chemical weapon component could interest, for example, the EPA, Chemical Safety Board and the FBI Counter-terrorism Division. Bottom Right Orange How many times you have to scrub your hands after touching it before they stop smelling weird (1) While the real NFPA 704 chart describes properties ranging from unsafe to potentially deadly, this square describes a minor but very real inconvenience. Some things are harder to wash off your hands than others, and, given that most people don't often work with dangerous substances,[citation needed] this would be a more common, but less relevant, concern for many people. In this case, the substance, or its residue, seems to be fairly easy to wash off. This is seemingly incongruous with its ratings in the Blue and Black squares (see below), though it's possible that this substance simply doesn't have a strong odor.
Bottom Black Number of times it's caused one of those terrifying lab accidents that chemists tell scary stories about late at night (2) This square might show how concerned and careful someone should be in handling the substance in question, especially if the number is more than one. However, it would be dependent not just on how inherently dangerous the substance is, but also on how commonly it occurs in labs. It's also vague as to what kind of accidents it has been involved in and what precautions therefore need to be taken. It could, for example, have caused some terrifying reaction, destroying things around it, or it could be very large and unwieldy and liable to crush people if handled improperly. In this case, it seems the substance has caused two such accidents, presumably on account of its high health risk of 4 in the Blue square, and may also be linked to its hazardous disposal score of 4 in the Purple square.