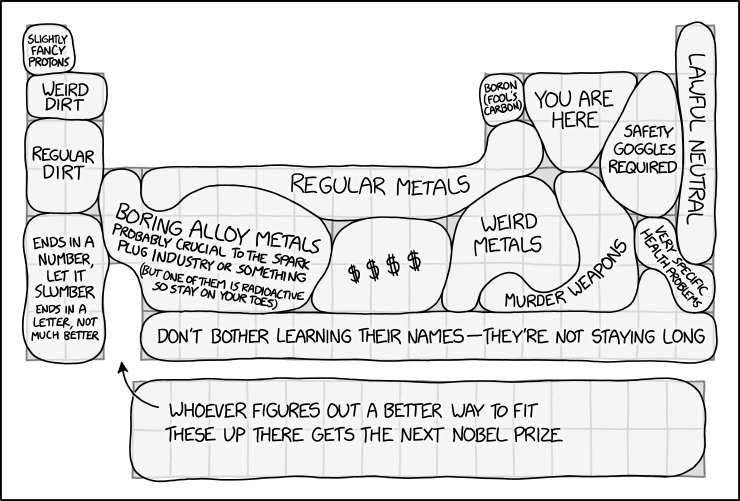
Periodic Table Regions
Cesium-133, let it be. Cesium-134, let it be even more.

Cesium-133, let it be. Cesium-134, let it be even more.
The periodic table is used to arrange chemical elements based on their properties. This comic groups them together into regions with labels humorously reflecting their properties, characteristics, or uses.
Table Sections
Section Real table Elements contained Explanation Slightly fancy protons Hydrogen Hydrogen Most hydrogen atoms (specifically of the isotope H-1, making up 99.9844% of all hydrogen on Earth) are a proton and an electron. Since the electron can be removed (so only a proton remains) and you can call that an H+ ion, Randall calls hydrogen atoms "slightly fancy protons". Weird dirt Lighter alkali and alkaline earth metals Lithium, Beryllium Lithium and beryllium, as some of the lightest elements, have unusual properties compared to heavier metals. Lithium, for instance, is the least dense metal on the periodic table and is used in applications such as rechargeable batteries and as a psychiatric medication. Beryllium is both toxic and transparent to X-rays, but also keeps its shape and stiffness over a wide range of temperatures, leading to its use in the primary mirrors of the James Webb Space Telescope. It was also used in F1, both in brake calipers and internal engine parts, before being outlawed (due to its toxicity). Also, both elements have nuclear applications. Regular dirt Middle alkali and alkaline earth metals Sodium, Magnesium, Potassium, Calcium Despite being metals, these are listed as "dirt" rather than "metal." Perhaps this is because they are commonly found in dirt, as they are essential nutrients for plant life and for many other forms of life, including humans. Ends in a number, let it slumber. Ends in a letter, not much better. Heavier alkali and alkaline earth metals Rubidium, Strontium, Cesium, Barium, Francium, Radium Highly reactive metals, some of which are commonly used as radioactive isotopes (which are known by a number; e.g. radium-223).
The title text mentions cesium-133 and cesium-134, with the former being the only stable isotope of cesium. The phrase, "cesium-133, let it be," in the title text is a reference to the mnemonic used to remind one how to identify and to avoid poison ivy: "leaves of 3, let it be". The joke is that these elements are so aggressively reactive that even where stable isotopes exist, they're incredibly dangerous to handle (ie, "not much better" than the radioactive ones).Boring alloy metals. It's probably crucial to the spark plug industry or something. (But one of them is radioactive so stay on your toes.) The left transition metals Scandium, Titanium, Vanadium, Chromium, Manganese, Yttrium, Zirconium, Niobium, Molybdenum, Technetium, Ruthenium, Hafnium, Tantalum, Tungsten, Rhenium, Osmium These elements tend not to be very well known to the general public, since they're rarely primary components in anything a typical person would encounter. Nonetheless, they're used as constituents (sometimes as a small but vital trace) in alloys with specific uses, including stainless steel, bulb filaments and superconductors.
A spark plug may use austenitic stainless steel, which includes chromium and (in some cases) molybdenum, for heat and oxidation resistance.
Technetium is the lightest element that has no stable isotope and is thus radioactive. Technetium is commonly used in medical imaging.Regular metals The top transition metals Titanium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Aluminum, Silicon Commonly known metals (and one metalloid, silicon). These all have important uses in construction and other major industries. Titanium is extremely lightweight and creates bright white sparks. Iron is a common building material. It is used in almost everything from bridges to buildings. Nickel and Zinc are both found in American coins (Zinc makes up 97.5% of the penny). Copper is part of Gold's family and is used mostly in wires because of its conducting properties. Aluminum is also extremely lightweight like Titanium. It is used in high-stress but lightweight applications such as bike frames and airplanes. $$$$ The platinum group Rhodium, Palladium, Silver, Osmium, Iridium, Platinum, Gold Rare and highly prized metals. The most expensive of these, osmium, is worth about $1,600 per gram as of when the comic was posted. Gold, silver, and platinum are famous for being precious metals, and are commonly used in jewelry. Weird metals The "ordinary metals" and some transition metals Gallium, Germanium, Cadmium, Indium, Tin, Mercury These are more obscure than the other metals (except tin and mercury) and tend to have fewer or more specialized uses. Mercury is also the only metal that is liquid at room temperature, and gallium melts just above that at 30 °C (86 °F). Indium is one of the only metals that can be chewed like bubble gum. This is because it is non-toxic and extremely soft. Boron (fool's carbon) Boron Boron Just as like how pyrite is commonly called "fool's gold", Randall calls boron "fool's carbon" due to its similarities in the way both elements can make stable covalently bonded molecules. Many of boron’s allotropes are also analogous with those of carbon. You are here Nonmetals Carbon, Nitrogen, Oxygen, Phosphorus Other than hydrogen, these are all the elements required to make DNA, and they make up the majority of atoms in other biological molecules, thus placing you over here. Murder weapons Ordinary metals and metalloids Arsenic, Antimony, Tellurium, Thallium, Lead, Bismuth, Polonium Arsenic, thallium, lead, and polonium are highly toxic and have notorious histories as poisons. Arsenic, specifically, was a frequently used poison for murders in the 19th century. A radioactive isotope of polonium has been used for clandestine state-sponsored murders, due to the tiny amount needed for a lethal dose. Antimony and tellurium are also hazardous, though to a lesser degree. Lead is also the primary metal used for making bullets, making it a potential tool for murder in a different way. Bismuth is the odd one out, having little toxicity at all, but it is used in lead-free bullets. Safety goggles required The lighter halogens + some of group 16 Fluorine, Sulfur, Chlorine, Selenium, Bromine These elements are highly reactive, so safety goggles are required. Randall has previously mentioned the nasty properties of bromine at room temperature in Extreme Boating and the awful things you can do with fluorine in Pressure Cooker. Very specific health problems Iodine and Radon Iodine, Radon Radon gas is formed in the radioactive decay series of uranium and thorium, which occur in trace levels in many common minerals. The gravel and concrete used in construction include such minerals, and the radon is released into the air via pores and cracks in the stone and concrete. The relatively poor ventilation in underground spaces such as basements and cellars can cause the radon to accumulate rather than be released into the environment. Eventually, the radon itself decays into other elements, which are also radioactive. Radon is chemically very inert and doesn't bind to anything, but it can still be inhaled, and its daughter elements can bind to dust particles. The radioactive materials, when inhaled, can cause damage to cells, especially in the lungs, with lung cancers as a possible long-term consequence. Iodine is a required nutrient that humans need in trace amounts to remain healthy, with an iodine deficiency typically causing thyroid problems such as goiter. Radioactive iodine is easily taken into the body, deliberately to counteract hyperthyroidism (by giving the thyroid gland radiation damage) or uncontrollably due to exposure to material in nuclear fallout/accidents. Giving high doses of 'normal' iodine would ideally flush out the problematic isotope. Even comparing the two radioactive effects, these two specific health problems are entirely unrelated, and it is only by coincidence that they are corner-to-corner on the periodic table. Lawful Neutral Noble gases Helium, Neon, Argon, Krypton, Xenon These elements are mostly non-reactive and are referred to as 'noble' as they typically do not associate with other elements. (The first three don't form chemical compounds at all, apart from things like unstable ionic complexes. The other two do form a few compounds, but these are rather difficult to synthesize and are quite reactive.) Lawful Neutral is a reference to the Dungeons & Dragons alignment chart, which gives moral categories for characters. The chart goes from Lawful to Chaotic on one axis, and Good to Evil on another. Lawful Neutral means following the law without any bias towards Good or Evil, which could be exemplified by the unreactivity of noble gases. See also: 2251: Alignment Chart Alignment Chart.
Don't bother learning their names – they're not staying long Astatine and Period 7 from Rutherfordium onwards Astatine, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium, Roentgenium, Copernicum, Nihonium, Flerovium, Moscovium, Livermorium, Tennessine, Oganesson These elements are hard to produce in large quantities and decay within hours or less... in some cases, milliseconds. (Their names haven't exactly been stable, either, with previous multiple systems of placeholder names. For example, dubnium has been called nielsbohrium, hahnium, joliotium, unnilpentium, and eka-tantalum.) Whoever figures out a better way to fit these up there gets the next Nobel Prize The internal transition metals Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium, Mendelevium, Nobelium, Lawrencium The lanthanides and actinides are usually placed disconnected from the main periodic table, largely because putting them where they "should" be would make the chart very long. Other types of periodic tables that arrange the elements exist; with the seventh period filled out the hunt is on for the eighth period which is expected to contain an extra 18 groups (columns), making a redesign even more prudent than ever. The periodic table of elements has previously been the subject in 2214: Chemistry Nobel, 2639: Periodic Table Changes, and 2723: Outdated Periodic Table. It is also referred to or indirectly referenced in a number of other comics, such as 18: Snapple, 821: Five-Minute Comics: Part 3, and 1052: Every Major's Terrible.